Stem Cells – Sources, Types, Applications, Concerns & Regulations
From Current Affairs Notes for UPSC » Editorials & In-depths » This topic
IAS EXPRESS Vs UPSC Prelims 2024: 80+ questions reflected
Stem cell research and its use in therapy are at the frontiers of biomedicine. It is being explored as a panacea for a wide range of diseases from tissue damages to serious conditions like neurodegenerative diseases and cancer. Most recently, it is being explored as a possible tool to treat patients with severe cases of COVID-19 in China. While it is a high potential tool, it also has its share of associated risks.
What is a stem cell?
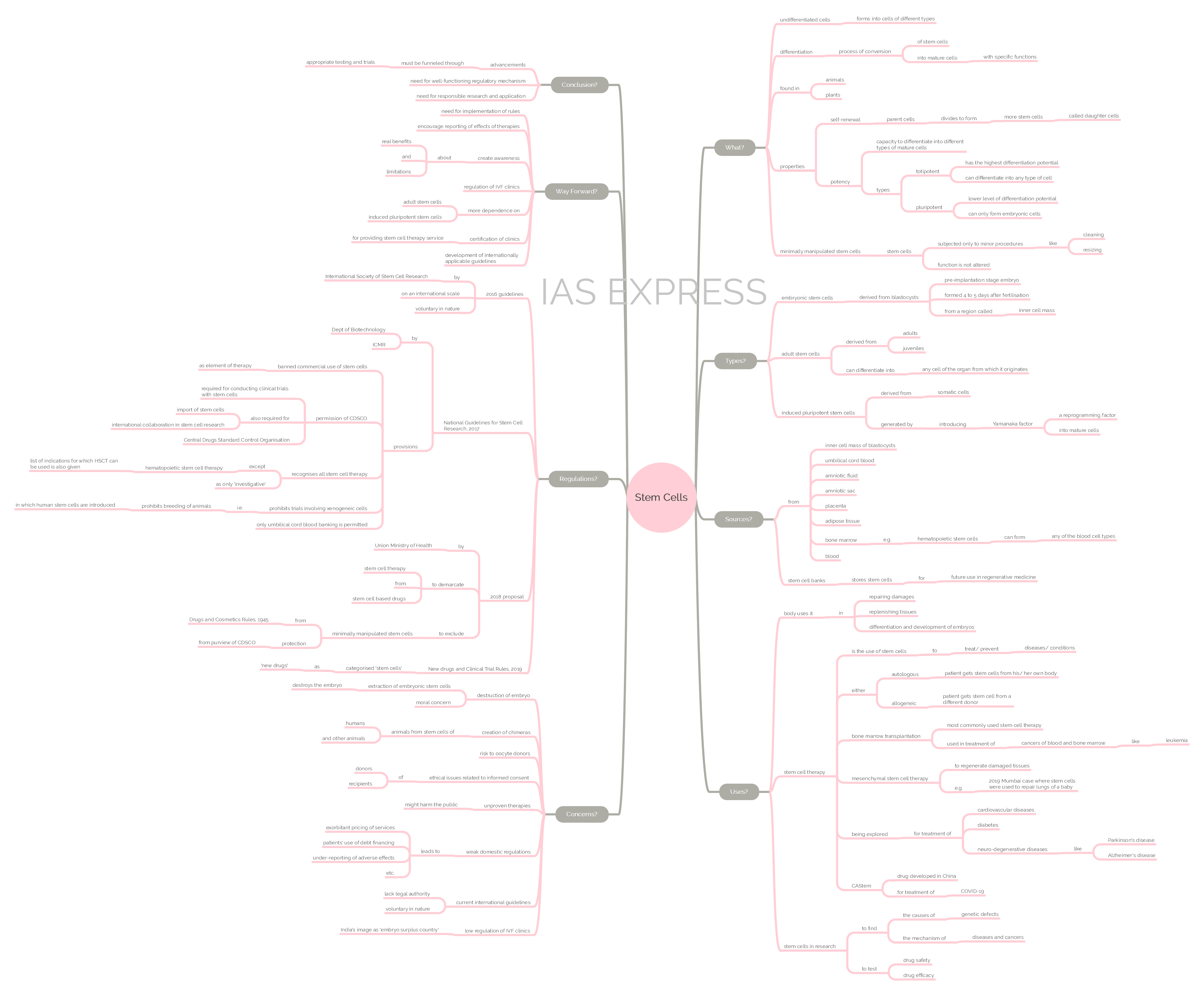
- Stem cells are undifferentiated cells that are capable of developing into other types of cells with specific functions. A normal cell in the body can undergo division to form only cells of its own type.
- These cells are present in both animals and plants.
- The process by which the stem cell is converted into a mature cell with a specific function is called differentiation.
- Two main properties of stem cells are:
- Self-renewal: The stem cells undergo cell divisions to form other stem cells and thus maintain their population. The dividing cell is called the parent cell and the resulting cells are called daughter cells.
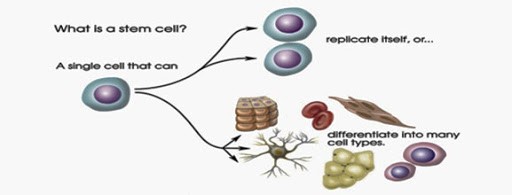
- Potency: The stem cells have different capacities to differentiate into mature cells i.e. potency. A stem cell can be totipotent (can produce all the cell types in a body and has the highest differentiation potential) or pluripotent (can differentiate only into any of the embryonic cells).
- Minimally manipulated stem cells are those that are subject to only minor procedures like cleaning and resizing but not any process that would alter their functions.
What are the types of stem cells?
There are different types of stem cells:
Embryonic Stem Cells:
- These are stem cells derived from the blastocyst stage of the embryo.
- The blastocyst is a pre-implantation stage, formed 4 to 5 days after fertilisation.
- They are pluripotent in nature and found in the inner cell mass.
Adult Stem Cells:
- Adult stem cells or somatic stem cells are found in both adult and juvenile animals, unlike embryonic stem cells.
- They have the ability to differentiate into any cell of the organ from which they originate.
Induced Pluripotent Stem Cells:
- These are stem cells that are derived from somatic cells.
- It is a pluripotent stem cell generated by introducing ‘reprogramming factors’ called Yamanaka factors into mature cells.
Where are the stem cells sourced from?
- Embryonic stem cells are derived from pre-implantation stage blastocysts’ inner cell mass. The extraction of the stem cell destroys the embryo.
- Stem cells are also harvested from umbilical cord blood, amniotic fluid, amniotic sac and the placenta.
- Somatic stem cells are sourced from blood, adipose tissue (fat) and bone marrow.
- Adult stem cells are preferred as their extraction doesn’t damage the host, unlike embryonic stem cells.
- One of the most important types of stem cells in the hematopoietic stem cell. These are found in the bone marrow and they give rise to different kinds of blood cells.
- Stem cell banking is used for storing stem cells for future use in regenerative medicines.
How are the stem cells used?
- The body uses the adult stem cells as a part of its repair system- to heal injuries and damages.
- It is also used to replenish the tissues.
- The embryonic stem cells are vital for the differentiation and development of the embryo.
- Stem cell therapy is a major application of stem cells. It is the use of stem cells in medicine to treat or prevent a disease/ condition.
- Stem cell therapy can be autologous (where the patient gets stem cells from his/her own body) or allogeneic (a donor donates stem cells to the patient).
- Bone marrow transplantation is the most widely used type of stem cell therapy. It is used for treating cancers of the blood or bone marrow, like leukemia.
- Stem cells are used to regenerate damaged tissues. In 2019, mesenchymal stem cell therapy was used to repair the lungs of a baby.
- It is being explored as an option for the treatment of cardiovascular diseases, diabetes and even neurodegenerative diseases like Parkinson’s and Alzheimer’s diseases.
- It is being used in research to find the causes of genetic defects, the mechanism behind cancers, various diseases, etc.
- It is also being used for testing the safety and efficacy of new drugs.
- The newly developed stem cell drug, CAStem, was reportedly successful in curing COVID-19 in animal experiments. This is because of its ability to promote the formation of new blood vessels, cell differentiation and multiplication and also inhibition of inflammatory response.
- Recently, China treated 4 COVID-19 patients with stem cell therapy on a trial basis. The patients had been discharged after recovery and the officials are now expanding the trial.
- Stem cell therapy was also used in treating H7N9 avian flu cases.
What are the concerns about the use of stem cells?
- The main issue in stem cell therapy and research is the controversy surrounding the use of embryonic stem cells. As the extraction process destroys the embryo, it poses a moral concern for those who believe that the fertilised embryo is to be treated as a human being.
- The creation of ‘chimeras’ or animals made from cells of different zygotes, or even different species has posed ethical concerns.
- There is a certain degree of risk to oocyte donors.
- Ethical issues related to informed consent of both, the donors and recipients of stem cells and products derived from it.
- There is a risk of harm from unproven stem cell therapies to the patients.
- Weak domestic regulation in some countries leads to commercialisation of stem cell therapy without the necessary transparency and scrutiny. Some of the consequences include exorbitant pricing of the service, use of debt financing by the patients, under-reporting of adverse effects, etc.
- Current international guidelines for stem cell research lack the political and legal authority to enforce regulations in the countries. Also, they are voluntary in nature.
- Low regulation of IVF clinics- which is being considered as an established source of embryos for extracting stem cells- including by foreign scientists. Growing concern about the international view of India as an ‘embryo surplus country’.
How is stem cell use regulated?
- Global regulation of stem cell research and therapy is still incomplete as there isn’t much legal backing for the existing guidelines.
- In 2016, the International Society of Stem Cell Research (ISSCR) released model voluntary guidelines for stem cell research.
- In 2017, the National Guidelines for Stem Cell Research was given by the Department of Biotechnology and the Indian Council of Medical Research.
- The guidelines banned the commercial use of stem cells as ‘elements of therapy’.
- The permission of the CDSCO (Central Drugs Standard Control Organisation) is required for conducting clinical trials using stem cell therapy.
- The 2017 guidelines recognised all stem cell therapies, apart from the hematopoietic stem cell therapy (HSCT), as only investigational at present. It also gave a list of indications for which HSCT can be used.
- The guidelines also prohibited clinical trials involving ‘xenogeneic cells’. breeding of animals in which human stem cells have been introduced is prohibited.
- The guidelines permit only umbilical cord blood banking.
- Import of stem cells and also international research collaborations in the field require CDSCO’s approval.
- In 2018, the ministry of health and family welfare proposed the exclusion of ‘minimally manipulated stem cells’ from the purview of the Drugs and Cosmetics Rules of 1945. This would classify these products as ‘therapy’ as opposed to ‘drugs’ and hence protect it from the screening and regulation by CDSCO. This would demarcate ‘stem cell-based drugs’ from ‘stem cell therapy’.
- The use of stem cells for therapy in India requires permission from the government. This is because stem cell-derived products are considered as ‘drugs’ under the New Drugs and Clinical Trial Rules, 2019, as of March, 2019.
What is the way forward?
- There is a need for implementation of the rules framed by the centre. Despite the existence of regulatory frameworks, scientists, clinics and doctors continue to work with stem cells without proper approval.
- There is a need to encourage reporting of the effects of stem cell therapies’ trials. Evidence-based progress is vital in a dynamic and much-hyped field like stem cell technology.
- There is a need to create awareness about the real benefits and limitations of stem cell therapies to protect patients from unproven therapies offered by profit-driven clinics.
- Regulation of IVF clinics is essential to break the image of India as an ‘embryo supplier’ for dubious research.
- Instead of depending on stem cells derived from embryos, adult stem cells or induced pluripotent stem cells can be worked with to a greater extent.
- Certification of clinics approved for providing stem cell therapy.
- An internationally applicable guideline must be developed by organisations like WHO as this is not an issue to be solved by one country in isolation.
Conclusion:
Though advancement in stem cell technology is making strides, there is a need to funnel it through appropriate testing and clinical trials before being used on the general public. For this, a well-functioning regulatory mechanism and responsible research and application are of vital importance.
Practice Question for Mains:
Stem cell therapy was one of the tools tested by the Chinese authorities to treat COVID-19 patients with a greater degree of success. Comment on the significance of this technology. (250 words).
If you like this post, please share your feedback in the comments section below so that we will upload more posts like this.