Quality in Pharma Sector- Lessons from Gambia Episode

From Current Affairs Notes for UPSC » Editorials & In-depths » This topic
IAS EXPRESS Vs UPSC Prelims 2024: 85+ questions reflected
What happened in Gambia?
- In July, Gambia’s Epidemiology and Disease Control Department took note of a sudden increase in cases of 5 months to 4 year old children showing acute kidney injury.
- By August, the situation had worsened to 32 cases and 28 deaths with a case fatality ratio of 87.5%.
- In September, the West African nation reported 4 Indian-made syrups– used for managing fever, cough and allergic cold- as the causative to the World Health Organization. These are: Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup, and Magrip N Cold Syrup– manufactured by Haryana-based Maiden Pharmaceuticals.
- WHO issued an alert after analysis of the syrups’ lab samples. Following this, Gambia started a country-wide recall of the syrups in October. Currently, the death toll has risen to 66.
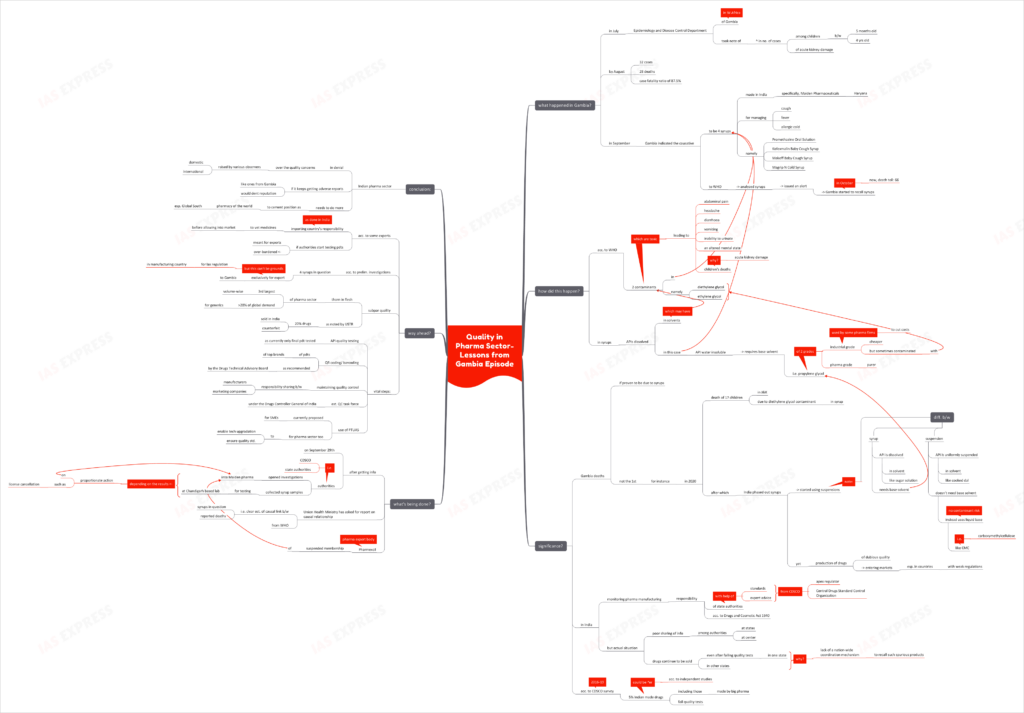
How did this happen?
- WHO confirmed the presence of unacceptably high amounts of 2 contaminants– diethylene glycol and ethylene glycol– in the syrups.
- In such syrups, the API or Active Pharmaceutical Ingredient is dissolved in a solvent. These are toxic chemical contaminants are found in the syrups’ solvents.
- Since the APIs, used in these 4 syrups-in-question, like paracetamol, are insoluble in water, a base solvent like propylene glycol is used. Some companies tend to use propylene glycol meant for industrial use (instead of the type meant for pharmaceutical use i.e. of higher purity) to cut costs. The industrial grade tends to contain diethylene glycol and ethylene glycol contaminants.
- These contaminants can lead to abdominal pain, headache, diarrhoea, vomiting, inability to urinate and an altered mental state. They are potentially fatal to children.
Why is this significant?
- If proven to be true, the Gambia deaths isn’t the 1st such case. In 2020, a syrup with diethylene glycol contamination led to the death of 17 children in Jammu and Kashmir.
- Following this incident, India had phased out syrups and shifted to administration of suspensions.
- Unlike syrups, that are like sugar solutions, suspensions are like cooked dal i.e. the API is uniformly suspended in a solvent. Suspensions don’t require propylene glycol as the API isn’t dissolved. Instead, a liquid base called CMC or carboxymethylcellulose, which has no risk of such contamination, is used.
- Despite this, dubious quality drugs continue to find their way into the market- especially the market of countries with weak regulations.
- State authorities are responsible for monitoring the manufacture and sale of drugs, according to the 1940 Drugs and Cosmetic Act. The CDSCO (Central Drugs Standard Control Organization) lays down the standards and provides expert advice to the state regulators. Yet, there is insufficient information-sharing between the state and central authorities, as seen from the 2020 incident.
- Drugs, failing quality tests in one state, continue to be marketed in other states. This is mainly due to the lack of a nation-wide coordination mechanism to recall such spurious products.
- According to a 2014-16 survey by the CDSCO, some 5% of Indian-made drugs, including those made by some big pharma firms, failed to pass the quality tests. Independent studies suggest that this percentage could be much higher.
What is being done?
- After receiving the information on September 29th, CDSCO and the state authorities opened investigations into Maiden Pharma’s syrups. Control samples were lifted and tested in a Chandigarh-based laboratory.
- Depending upon the results of this investigation, the company is to be subjected to proportionate action, including license suspension.
- The Union Health Ministry has requested for a report on the causal relationship from WHO. This is to undoubtedly establish that it was indeed the syrups that led to each and every death reported.
- Following WHO’s alert, Pharmexcil (pharmaceutical export body) suspended the membership of Maiden Pharma.
What is the way ahead?
- Some experts argue that it is the importing country’s responsibility to vet medicines before releasing them into the market. This is something even India does. However, if the Indian authorities were to start testing even the exported medicines, they would be over-burdened.
- Preliminary investigations have revealed that these 4 syrups were manufactured exclusively for export to Gambia. Yet, this shouldn’t be grounds for relaxing regulatory oversight over such products in the country of manufacture.
- Already, subpar pharmaceuticals is a thorn in the flesh for the Indian pharmaceutical story. According to USTR (Trade Representative), some 20% of drugs sold in India is counterfeit.
- This percentage holds major significance for India which is the 3rd largest drug manufacturer (in terms of volume) and meets more than 20% of the global demand for generics.
- Hence some steps are vital to safeguard India’s position as the pharmacy of the world:
- Quality testing of APIs too- as the current practice is to simply test the final product
- QR coding or barcoding of products from the top brands, as recommended by the Drugs Technical Advisory Board
- Maintaining quality control, with the responsibility being shared between both- the manufacturers and the marketing companies
- Establishing a quality control task force under the Drugs Controller General of India
- Use of the PTUAS (Pharmaceutical Technology Upgradation Assistance Scheme), currently proposed for SMEs, for pharma companies to enable technological upgradation and ensure quality standards
Conclusion:
The Indian pharma sector has been in denial over the quality concerns raised by various observers- both domestic and international. If adverse reports on the Indian pharma sector, such as the ones from Gambia, continue, then the sector’s hard-earned reputation could take a major dent. There is a need to do more in cementing India’s position as the pharmacy of the world, especially the Global South.
Practice Question for Mains:
How is quality control a major challenge to India’s position as the world’s pharmacy? Discuss with reference to the Gambia deaths. (250 words)
If you like this post, please share your feedback in the comments section below so that we will upload more posts like this.

