Femtoscope
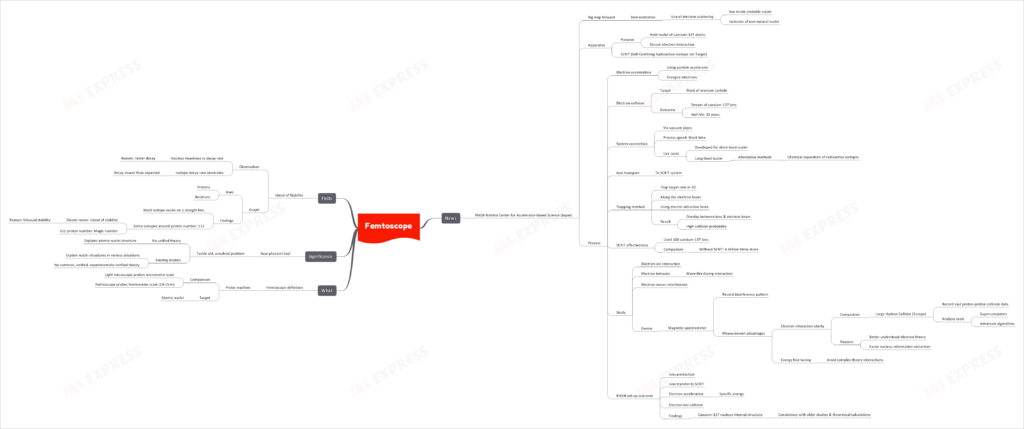
The RIKEN Nishina Center for Accelerator-Based Science in Japan has made significant advancements with the demonstration of the use of electron scattering to peer inside unstable nuclei. This breakthrough involves the inclusion of non-natural nuclei, opening a new dimension in the study of atomic structures.
Femtoscope Explained
What is a Femtoscope?
- A probe machine designed to investigate on an incredibly minute scale.
- Comparison:
- Light microscopes can probe at the micrometre scale.
- The Femtoscope delves into the femtometer scale (10^-15 m).
- Comparison:
- Primary Target: Atomic nuclei.
The Breakthrough at RIKEN
- Apparatus:
- SCRIT (Self-Confining Radioactive-isotope Ion Target) system holds nuclei of caesium-137 atoms ensuring electron interaction.
- Process:
- Accelerate electrons using a particle accelerator to energize them.
- Electrons then collide with a block of uranium carbide, producing a stream of caesium-137 ions with a half-life of 30 years.
- These ions are transported to the SCRIT system.
- A trapping method is used where target ions are trapped in a 3D space along the electron beam using an electric attractive force.
- This results in a high probability of collisions between ions and the electron beam.
- SCRIT’s effectiveness is notable, using only 108 caesium-137 ions as opposed to a trillion times more without SCRIT.
- Study Outcome:
- Electron-ion interaction and behavior of electrons (wave-like during interaction) were studied.
- Electron-waves interference was observed using a magnetic spectrometer to record the interference pattern.
- Comparatively, the clarity of electron interactions in the RIKEN setup is greater than the Large Hadron Collider’s vast proton-proton collision data in Europe.
- Results:
- The internal structure of the Caesium-137 nucleus was found to be consistent with previous studies and theoretical calculations.
Significance of the Femtoscope
- A new tool for physicists that could help:
- Address the age-old challenge of not having a unified theory explaining the structure of atomic nuclei.
- While there are existing models that describe nuclei structures in various scenarios, there isn’t a universally accepted, experimentally-verified theory.
Interesting Facts: The Island of Stability
- Observation:
- Generally, the heavier a nucleus is, the faster it decays.
- Some isotopes, however, decay slower than expected.
- Graph Findings:
- While most isotope nuclei can be plotted on a straight line, some isotopes centered around the proton number 112 cluster in an area referred to as the “Island of stability” due to their unusual stability. This proton number, 112, is thus labeled the “magic number.”
If you like this post, please share your feedback in the comments section below so that we will upload more posts like this.

