India’s Updated GMP for Drug Makers
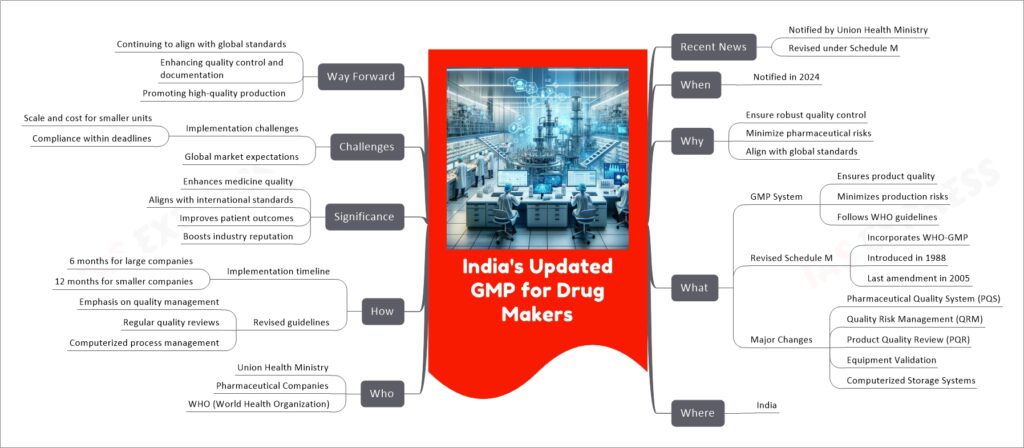
India’s Updated Good Manufacturing Practices (GMP) for Drug Makers, notified in 2024 by the Union Health Ministry, mark a significant revision of the existing standards under Schedule M of the Drugs and Cosmetics Rules. The update aims to ensure robust quality control in pharmaceutical production, aligning India’s standards with global norms, especially those of the World Health Organization (WHO). Major changes include the introduction of a Pharmaceutical Quality System (PQS), Quality Risk Management (QRM), Product Quality Review (PQR), validation of equipment, and computerized storage systems. The revisions require large companies to implement these changes within six months and smaller companies within a year. This move is expected to elevate the quality of medicines, improve patient outcomes, and enhance the reputation of the Indian pharmaceutical industry on a global scale.
If you like this post, please share your feedback in the comments section below so that we will upload more posts like this.