Pharmacopoeial Discussion Group
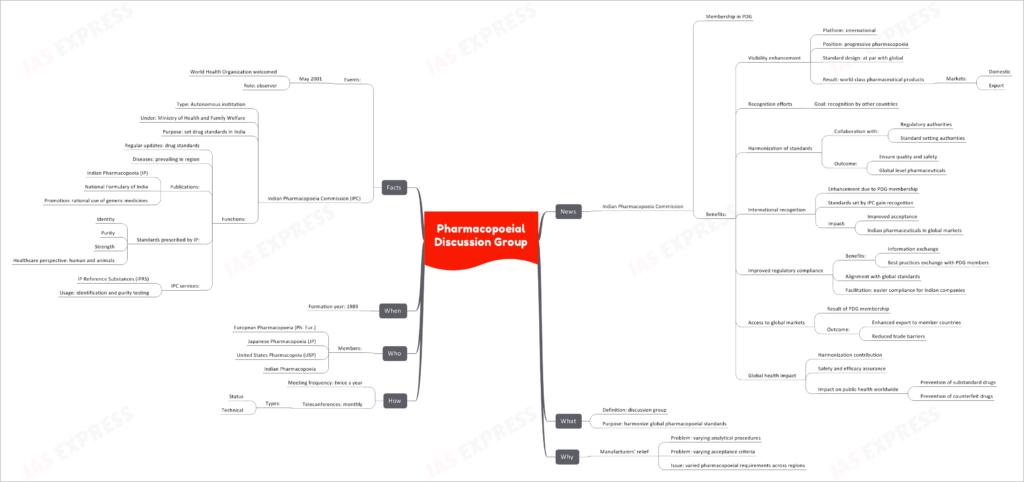
In the realm of pharmaceuticals, the Pharmacopoeial Discussion Group (PDG) plays a pivotal role in harmonizing global pharmacopoeial standards. This international collaboration, involving key players like the Indian Pharmacopoeia Commission (IPC), aims to enhance visibility, recognition, and regulatory compliance in the pharmaceutical industry.
Membership in PDG
Indian Pharmacopoeia Commission’s Involvement
The Indian Pharmacopoeia Commission (IPC) is a proud member of the PDG. Membership in this prestigious group brings several benefits:
- Visibility Enhancement: IPC gains an international platform as part of a progressive pharmacopoeia movement. Its standards align with global counterparts, resulting in the production of world-class pharmaceutical products for both domestic and export markets.
- Recognition Efforts: IPC strives for recognition by other countries, focusing on harmonizing standards with the collaboration of regulatory and standard-setting authorities. This effort ensures the quality and safety of pharmaceuticals at a global level.
- International Recognition: PDG membership enhances IPC’s international recognition. The standards set by IPC gain acceptance worldwide, leading to improved acceptance of Indian pharmaceuticals in global markets.
- Improved Regulatory Compliance: IPC benefits from information and best practices exchange with PDG members. Alignment with global standards facilitates easier regulatory compliance for Indian pharmaceutical companies.
- Access to Global Markets: PDG membership results in enhanced exports to member countries and reduced trade barriers, ultimately expanding the reach of Indian pharmaceutical products.
- Global Health Impact: IPC’s contribution to harmonization assures safety and efficacy, preventing substandard and counterfeit drugs. This has a significant impact on public health worldwide.
What is PDG?
Definition and Purpose
The Pharmacopoeial Discussion Group (PDG) is essentially a discussion group formed with the primary purpose of harmonizing global pharmacopoeial standards. It serves as a platform for discussions and collaborations to achieve uniformity in pharmaceutical standards across regions.
Why is PDG Necessary?
Manufacturers’ Relief
PDG addresses critical issues faced by pharmaceutical manufacturers:
- Varying Analytical Procedures: Different regions often have varying analytical procedures for the same drug, leading to confusion and inefficiency.
- Varying Acceptance Criteria: Divergent acceptance criteria for drug quality assessment can be challenging for manufacturers.
- Varied Pharmacopoeial Requirements: PDG seeks to overcome the challenges posed by varying pharmacopoeial requirements across different regions, ensuring consistency in drug standards.
How Does PDG Operate?
Meeting Frequency and Teleconferences
- PDG convenes twice a year to discuss and deliberate on pharmacopoeial standards.
- Monthly teleconferences, comprising both status and technical discussions, facilitate ongoing collaboration among members.
Who are the Key Players?
PDG Member Organizations
PDG consists of several prominent pharmacopoeias, including:
- European Pharmacopoeia (Ph. Eur.)
- Japanese Pharmacopoeia (JP)
- United States Pharmacopeia (USP)
- Indian Pharmacopoeia (IPC)
When Did PDG Originate?
Formation Year
The Pharmacopoeial Discussion Group (PDG) was established in the year 1989 to address the growing need for harmonized pharmacopoeial standards.
Additional Facts
Notable Events and IPC’s Role
- In May 2001, the World Health Organization (WHO) welcomed PDG as an observer, recognizing its significant contributions to global pharmaceutical standardization.
- The Indian Pharmacopoeia Commission (IPC) operates as an autonomous institution under the Ministry of Health and Family Welfare in India. Its primary purpose is to set drug standards in the country.
- IPC’s functions include regular updates to drug standards, addressing prevalent diseases in the region, and publishing documents like the Indian Pharmacopoeia (IP) and the National Formulary of India. IPC also promotes the rational use of generic medicines.
- Standards prescribed by IP encompass aspects such as identity, purity, strength, and healthcare perspectives for both humans and animals.
- IPC provides essential services like IP Reference Substances (IPRS) for identification and purity testing in the pharmaceutical industry.
If you like this post, please share your feedback in the comments section below so that we will upload more posts like this.